Nematodes are unsegmented roundworms, different from earthworms and other familiar worms that are segmented (annelids) or, in some cases, flattened and slimy (flatworms). Many kinds of nematodes may be found in the soil of any landscape. Most are beneficial, feeding on bacteria, fungi, or other microscopic organisms. Some nematodes are used as biological controls to help manage important insect pests. Plant-parasitic nematodes are nematodes that feed on live plants (Figure 1).
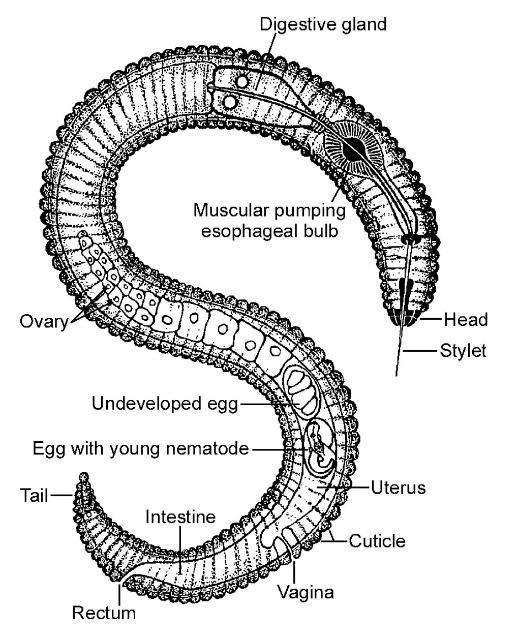
Figure 1. Diagram of a generic plant-parasitic nematode.
Credit: R. P. Esser, Florida Department of Agriculture and Consumer Services, Division of Plant Industry; used with permission.
WHAT ARE NEMATODES?
Nematodes are unsegmented roundworms, different from earthworms and other familiar worms that are segmented (annelids) or, in some cases, flattened and slimy (flatworms). Many kinds of nematodes may be found in the soil of any landscape. Most are beneficial, feeding on bacteria, fungi, or other microscopic organisms. Some nematodes are used as biological controls to help manage important insect pests. Plant-parasitic nematodes are nematodes that feed on live plants (Figure 1).
Plant-parasitic nematodes are very small, and most can only be seen using a microscope (Figure 2). All plant-parasitic nematodes have a stylet (or mouth-spear) that is similar in structure and function to a hypodermic needle (Figure 3). The stylet is used to puncture plant cells and then inject digestive juices and ingest plant fluids. Most of the plant-parasitic nematodes that are important on ornamental plants in Florida feed on roots. Some plant-parasitic nematodes, called “ectoparasites,” remain in the soil and feed by inserting only their stylet into the root (Figure 4). Other nematodes enter the plant with part or all of their bodies and are called “endoparasites.” Some endoparasites, called “migratory endoparasites,” continually burrow around inside the root (Figure 5). Other endoparasites, called “sedentary endoparasites,” establish permanent feeding sites inside the root and remain in one place. As it matures, a sedentary endoparasite’s body changes shape, and adult females are usually swollen (Figure 6).
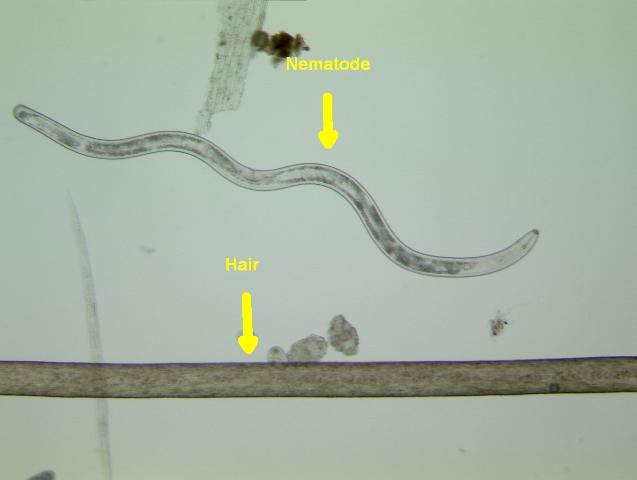
Figure 2. Size of a lance nematode (one of the larger plant-parasitic nematodes) compared to a human hair.
Credit: W. T. Crow, UF/IFAS
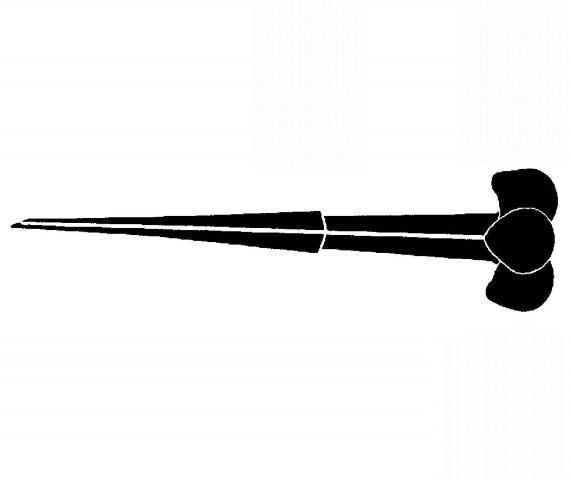
Figure 3. Diagram of a plant-parasitic nematode stylet.
Credit: R. P. Esser, Florida Department of Agriculture and Consumer Services, Division of Plant Industry; used with permission.

Figure 4. Ectoparasitic nematodes from a soil sample.
Credit: W. T. Crow, UF/IFAS
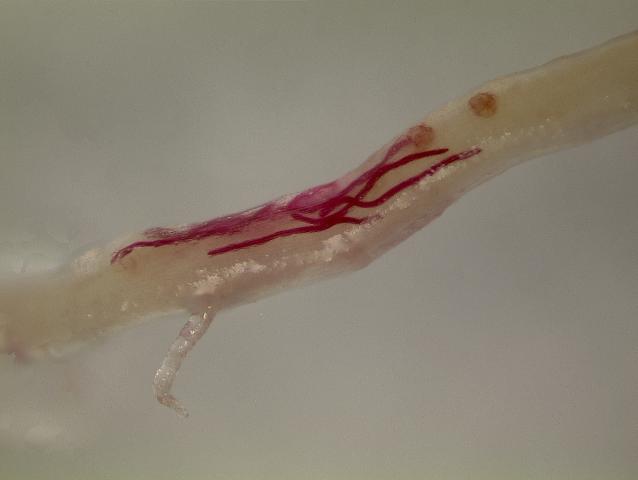
Figure 5. Migratory endoparasitic nematodes (stained red) tunneling within a root.
Credit: A. C. Hixson, UF/IFAS
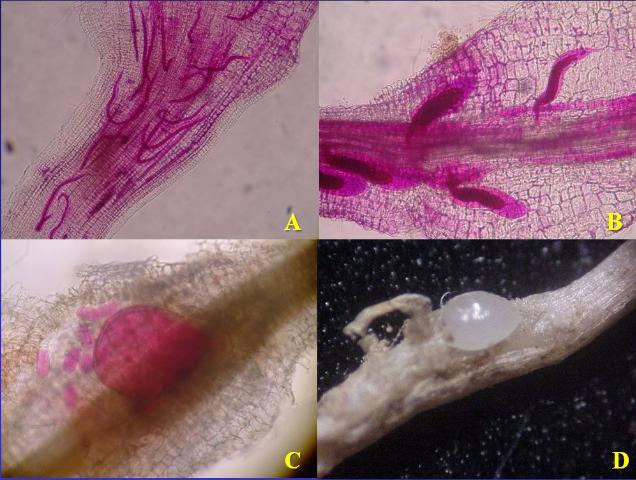
Figure 6. Development of sedentary endoparasitic root-knot nematodes within roots. (A.) Second-stage juveniles enter root, cause a feeding site, and then no longer move. (B.) Juveniles swell and molt several times. (C.) Adult female nematode is swollen and starting to lay eggs. (D.) Root tissue pulled back to show adult female nematode.
Credit: Panels A, B, and C, N. A. Sikora UF/IFAS; Panel D, Theresa Friday UF/IFAS Extension
HOW DO NEMATODES DAMAGE PLANTS?
Plant-parasitic nematodes damage the root system and reduce the ability of the plant to obtain water and nutrients from the soil. When nematode numbers increase, and/or when environmental stresses occur, aboveground symptoms may become evident. Aboveground nematode symptoms may resemble nutrient deficiencies or drought stress. Symptoms include yellowing (Figure 7), wilting, thinning (Figure 8), stunting (Figure 9), or dying. Nematode damage usually occurs in localized areas that may enlarge slowly over time. In a hedge, a few affected plants may be surrounded by healthy ones. Be aware that similar conditions may be caused by other factors, such as localized soil conditions, fungal diseases, or insects.

Figure 7. Nematode-infected gardenia (left) is stunted and yellow.
Credit: R. A. Dunn, UF/IFAS
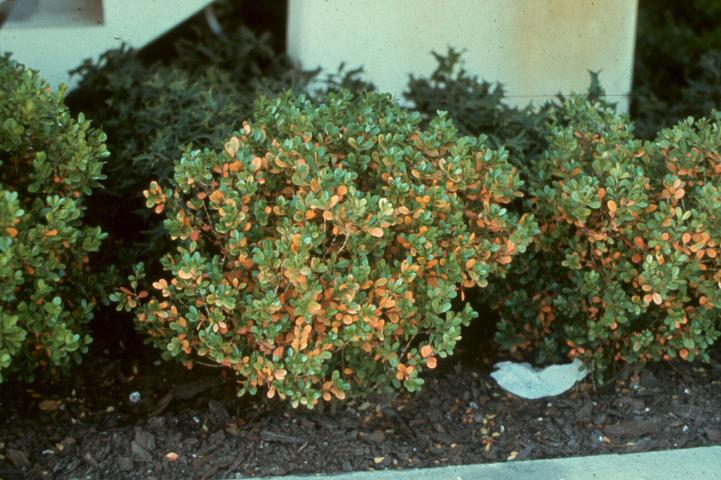
Figure 8. Nematode-infected landscape plants slowly lose leaves and thin over time.
Credit: R. A. Dunn, UF/IFAS

Figure 9. Nematode-infected pittosporum (four plants on left) are stunted compared to non-infested plants (two plants on right).
Credit: R. Baidoo, UF/IFAS
On trees and shrubs, root nematode damage usually becomes evident over a period of months or years. The plant canopy will get progressively thinner as leaves are shed. If a plant dies rapidly with the leaves still attached, nematodes usually are not the primary problem.
ROOT-KNOT NEMATODES
Root-knot nematodes are the most well known of the plant-parasitic nematodes. These are sedentary endoparasites. Four species are common in Florida on perennial landscape plants: Meloidogyne incognita, M. arenaria, M. javanica, and M. enterolobii, although other species also occur less frequently. Root-knot nematodes inject hormones into the roots that cause knots or galls to form (Figure 10). These galls are one of the few nematode symptoms that are easily recognized. Root-knot nematodes cause extensive physical and chemical changes in the plant that can make it more susceptible to fungal and bacterial pathogens. Some of these plant pathogens cause rotting of the root systems, and others cause vascular wilts. Sometimes the damage caused by the nematodes and the other organisms together is worse than that caused by either organism separately.
Root-knot nematodes are one of the most destructive plant-parasites, especially in sandy soil. High infestations can kill many types of perennial landscape plants. However, not all plants are affected equally by different kinds of nematodes. Table 1 has a list of perennial landscape plants that are commonly damaged by root-knot nematodes in Florida. This is not an exhaustive list; most kinds of plants may be affected by root-knot occasionally.

Figure 10. Galls or knots on liriope roots caused by root-knot nematodes.
Credit: W. T. Crow, UF/IFAS
OTHER SEDENTARY ENDOPARASITES
Several genera of nematodes are sedentary endoparasites that occasionally damage ornamental plants in Florida. These nematodes do not cause galls like root-knot nematodes. The most typical symptom is an unthrifty root system.
Reniform nematodes are limited to soils with high silt content. In Florida they are found most often in the Miami-Dade County area and parts of the Panhandle. Reniform nematodes are damaging to some ornamental plants and tropical fruits in the limited areas where they occur.
In Florida, the citrus nematode is a common problem on citrus but also can damage olives and persimmons. This nematode normally does not kill trees but causes them to be unthrifty and have reduced fruit production. This nematode causes a disease known as “slow decline” of citrus.
Several genera of nematodes are sedentary endoparasites that occasionally damage ornamental plants in Florida. These nematodes do not cause galls like root-knot nematodes. The most typical symptom is an unthrifty root system.
Reniform nematodes are limited to soils with high silt content. In Florida they are found most often in the Miami-Dade County area and parts of the Panhandle. Reniform nematodes are damaging to some ornamental plants and tropical fruits in the limited areas where they occur.
In Florida, the citrus nematode is a common problem on citrus but also can damage olives and persimmons. This nematode normally does not kill trees but causes them to be unthrifty and have reduced fruit production. This nematode causes a disease known as “slow decline” of citrus.
MIGRATORY ENDOPARASITES
Lesion nematodes are migratory endoparasites common in Florida soils. These nematodes usually cause dark, sunken areas called “lesions” on roots (Figure 11). Lesion nematodes are particularly damaging to ferns, bulbs, and citrus, but some species can damage other perennial plants as well. The coffee lesion nematode causes “citrus slump,” a devastating disease of citrus (Figure 12).

Figure 11. Dark, sunken lesions on amaryllis roots caused by lesion nematodes.
Credit: W. T. Crow, UF/IFAS

Figure 12. Citrus slump, caused by coffee lesion nematode.
Credit: R. A. Dunn, UF/IFAS
Burrowing nematodes are very damaging but not widespread in Florida. Citrus nursery stock are regularly inspected for this nematode, which is a regulated pest in Florida. “Spreading decline” is the disease of citrus caused by burrowing nematodes. Burrowing nematodes cause lesions and rotting of roots (Figure 13). In Florida, these nematodes are damaging to citrus, banana and its relatives, palms, and other ornamental plants.

Figure 13. Rotting roots caused by burrowing nematode.
Credit: R. A. Dunn, UF/IFAS
ECTOPARASITES
Several ectoparasitic nematodes are capable of causing damage to many landscape plants in Florida. The most destructive of these are the sting, awl, and stubby-root nematodes. Feeding by these nematodes usually causes a root system to be stunted or stubby-looking (Figure 14).

Figure 14. Abbreviated, stubby roots caused by ectoparasitic nematodes.
Credit: W. T. Crow, UF/IFAS
Sting nematodes are found in sandy soil and are common throughout much of Florida. Awl nematodes usually are found in wet habitats such as near ditches, ponds, or poorly drained areas. Several stubby-root nematode species are found in Florida, and one or more usually can be found in most Florida habitats. Other ectoparasitic nematodes that parasitize some landscape plants are spiral, ring, stunt, sheath, sheathoid, dagger, and needle nematodes.
VASCULAR AND FOLIAR NEMATODES
While most plant-parasitic nematodes feed on plant roots, there are a few that feed on the leaves or stems of some plants.
Pine wilt nematode (Bursaphelenchus xylophilus) infects pine trees. These nematodes are spread by certain beetles. The nematodes plug up the vascular system and reduce the flow of water up to the foliage. The pine needles droop, turn brown, and die. Pine wilt nematode is native to the United States, and our native pines are usually tolerant of them. However, in times of drought, pine wilt nematodes will cause additional stress to the trees. Pine species that originate in Asia and Europe, where pine wilt nematodes are not native, are very susceptible to pine wilt nematodes. Susceptible pines include Scotch pine, Austrian pine, Japanese black pine, mugo pine, and Japanese red pine.
Red ring nematode or coconut nematode (Bursaphelenchus cocophilus) is very damaging to coconut and oil palms. These nematodes are also spread by certain beetles. This nematode causes major problems for coconut production throughout the Caribbean region but is not yet present in Florida. If this nematode does get into Florida, it could cause serious damage to our coconut and certain other palms.
Foliar nematodes (Aphelenchoides spp.) infect leaf and bud tissue of many kinds of plants. They cause angular spots on leaves (Figure 15) that are similar to those caused by bacterial leaf spot pathogens. In landscapes, these are typically problematic only in shady areas with poor air movement.

Figure 15. Angular leaf spots caused by foliar nematodes.
Credit: W. T. Crow, UF/IFAS
HOW DO I KNOW IF NEMATODES ARE A PROBLEM?
With any plant problem, an accurate diagnosis is important to address the problem and to avoid wasting effort and making unnecessary pesticide applications. Generally, nematode symptoms are similar to other disorders, so visual inspection is not enough. The only reliable way to determine if plant-parasitic nematodes are involved in a plant problem is by having a nematode assay conducted by a professional nematode diagnostic lab. The University of Florida Nematode Assay Lab (NAL) is such a facility. See the NAL website at http://entnemdept.ufl.edu/nematology-assay-lab/ for information on forms, fees, and sample submission. The NAL staff will assign a level of risk to the sample based on what kind of nematodes are found, how many of them there are, and the type and age of the plants involved. To make an accurate nematode diagnosis, the lab must have a quality sample. Whether you are submitting a sample to the Florida Assay Lab or another lab, following the guidelines below can help ensure that the results will be as accurate as possible.
SAMPLING
Sometimes samples are collected before planting to find out if nematodes are a potential problem and, if so, to take protective measures. Pre-plant samples will typically only consist of soil. After planting, samples are collected from sick plants to determine if nematodes are the cause of plant decline. These samples require both soil and roots.
Before planting: Collect soil from 8 to 12 locations in the area where you intend to plant. Samples should be taken about 6 to 8 inches deep. If the soil is dry, dig down to where there is some soil moisture to collect the samples, and do not include the dry soil. A small handful of soil from each location is adequate. Combine all the soil into a single plastic bag. The total volume of soil from the samples should be about 1 pint. Samples may be taken with a shovel, trowel, or other device. If using a shovel, you can put part of the soil from 8 to 12 shovels full into a bucket. Thoroughly mix the soil in the bucket, and then take out a pint to submit for analysis.
After planting: Often a nematode assay is needed to determine if nematodes are causing a plant to get sick. For this type of sample, both soil and roots are required. Dig soil and roots from underneath the canopy of the symptomatic plants. Soil should be collected from where most of the fibrous roots are. Usually six inches deep is sufficient. Collect roots that are less than 1/4 inch in diameter. If multiple plants are affected, collect some soil and roots from several plants. Place the soil and roots together in the same plastic bag. A minimum of 1 pint of soil and 1 to 2 cups of roots are required.
Handling:
- Nematode samples should be kept in plastic bags to keep the sample moist. Do not send samples in soil test bags! Soil testing bags are designed to dry soil out. Dried-out samples are no good for nematode diagnosis. If using a self-sealing bag, tape the seal shut so that the bag does not come open in the mail.
- If submitting more than one sample, make sure that the outside of each bag is labeled with a permanent marker. You can also write on masking tape stuck to the bag. Do not put paper labels inside of bags, because they decompose and become illegible.
- Keep samples out of direct sunlight or heat. Heat and ultraviolet light kill nematodes. Even a few minutes on the dashboard or in the back of a pickup can invalidate assay results. Keep the samples in an air-conditioned room until they can be shipped.
- Handle the sample gently and pack it well. The more the soil gets banged around, the more the nematodes may get destroyed.
Fill out the information on the forms with as much information as possible. The diagnostician needs detailed information about the type of plant or plants in question and the cultivar, if known. Make sure that the information on the form matches the identification on the sample bag (i.e., front yard, back yard).
WHAT CAN I DO ABOUT NEMATODES?
CHEMICAL NEMATICIDES
There are no chemical nematicides labeled for use on landscape plants that have been effective in University of Florida trials.
BIONEMATICIDES
The bionematicide MeloCon WG contains a fungus that parasitizes nematode eggs. Results from University of Florida trials indicate that MeloCon can suppress but not eliminate nematodes on shallow-rooted perennial plants. When purchasing MeloCon, have it delivered directly to you from the manufacturer, and keep it frozen until it is used. To apply, mix MeloCon with water, and then either drench or spray it onto the soil around the base of the plant(s).
ORGANIC AMENDMENTS
Organic amendments can be added to soil as compost, manure, mulch, or other materials. Organic matter can help prevent nematode damage in several ways. The organic matter increases the ability of the soil to hold water and nutrients and to improve soil structure. This makes a better environment for most plants and can help the plants survive in spite of the nematodes. Organic amendments also can increase natural enemies of nematodes that suppress the nematode populations. Some organic amendments can release chemicals or gases that are toxic to the nematodes.
There are several organic nematode management products for sale. Researchers with the University of Florida have worked with a number of these, but probably not all of them. In the majority of cases, these products work no better than adding any other, less expensive, organic material.
For more detailed information on these topics see Soil Organic Matter, Green Manures, and Cover Crops for Nematode Management online at https://edis.ifas.ufl.edu/vh037 or at your local UF/IFAS Extension office.
CULTURAL PRACTICES
There are many steps you can take to avoid, or at least minimize, nematode interference with growing perennial plants. Combine as many of these steps as possible into an integrated nematode management program to get the best chance of managing a nematode problem successfully. Some of these tactics may seem futile or even silly, but each can make a real contribution to suppression of nematode problems.
- Pest exclusion is the most important strategy to prevent nematode problems. Sometimes plants come from the nursery already infested with nematodes. Avoid introducing nematodes that you do not have on your property already by carefully inspecting plant roots before planting. Do not use plants whose roots have galls (Figure 16), are rotten, or have other abnormalities.
- Do not plant a crop that is highly susceptible to one or more kinds of nematode present on your site (Table 1).
- If you must plant in areas with high risk of nematodes for your crop, consider soil replacement, which requires you to dig out and remove all soil from a generous planting hole (at least 3 ft diameter × 2 ft deep) and replace it with soil free of nematodes and other pests. The crop roots eventually will grow out of the clean soil volume and there will be some movement of nematodes into the clean soil, but the plant will have a jump-start on them by having some time to grow nematode-free.
- Soil amendments are any organic materials that might be added to soil to improve its physical, chemical, or biological characteristics (https://edis.ifas.ufl.edu/vh037). Compost or raw manure, leaves, or other organic products provide benefits for fruit tree growth and help trees tolerate nematodes better. The amendments should be incorporated into the planting hole for new plantings and added to the soil surface for established plants.
- Soil solarization (https://edis.ifas.ufl.edu/in856) involves covering soil with clear polyethylene for an extended period in the summer (Figure 17). Solarization of field planting sites in Florida may be disappointing, because it rarely heats soil sufficiently at the recommended depth (5–6 inches is common) to provide adequate control for the entire root zone area. However, a limited volume of otherwise good topsoil or soil mix can be solarized by completely enclosing it in clear polyethylene film and keeping the soil depth at no more than 6 inches. The soil should be located to receive full sun all day, and should be exposed 6 weeks or longer in June and July. This might be a good way to reduce nematodes and fungi problems in existing soil or in a soil mix to be used in soil replacement.

Figure 16. Root-knot nematode galls on roots of a container-grown plant.
Credit: R. A. Dunn, UF/IFAS

Figure 17. Soil solarization in a landscape.
Credit: R. A. Dunn, UF/IFAS
SUMMARY
Following the recommendations listed in this document can help avoid or reduce problems with plant-parasitic nematodes in the landscape. However, there are no guarantees of success. County and state faculty with the University of Florida are continually exploring new nematode-management options. This document will be updated periodically to make the most recent data available to you. If you are reading an older version of this document, you are encouraged to look at the current version online at https://edis.ifas.ufl.edu/ or at your local UF/IFAS Extension.
TABLES
Table 1.
Perennial landscape plants commonly damaged by root-knot nematodes in Florida as diagnosed by the University of Florida Nematode Assay Lab.
FOOTNOTES
The use of trade names in this publication is solely for the purpose of providing specific information. UF/IFAS does not guarantee or warranty the products named, and references to them in this publication do not signify our approval to the exclusion of other products of suitable composition. All chemicals should be used in accordance with directions on the manufacturer’s label. Use pesticides safely. Read and follow directions on the manufacturer’s label.

